Abstract
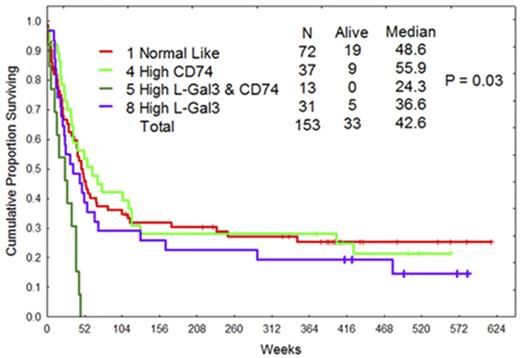
Galectin 3 (LGALS3) is a glycan binding protein that positively regulates survival signaling by diverse mechanisms. LGALS3 is involved in trafficking of kinases such as RAS and receptors such as CD44 to membranes. LGALS3 gene expression is associated with poor survival in AML (Cheng et al Blood 2013) but its functional role is not clear. We therefore analyzed protein expression of LGALS3 and 230 other proteins by RPPA in 511 AML patients. LGALS3 was significantly elevated in AML blast cells compared to normal CD34+ counterparts and highest in monocytic AML patients. LGALS3 levels were highest in Intermediate and Unfavorable Cytogenetic groups. Pearson correlation of LGALS3 with the other proteins identified a distinct set of 23 proteins positively and 13 proteins negatively correlated with LGALS3 expression levels. LGALS3 positively correlated with the expression of anti-apoptotic BCL2 family member MCL-1. To validate RPPA results, OCI-AML3 cells were transduced with a lentivirus expressing shRNA against LGALS3 or with a control lentivirus expressing shRNA against a non-human gene (i.e. GFP). Suppression of LGALS3 in the cells results in reduced MCL-1 gene and protein expression. As reduction of LGALS3 by shRNA or inhibition of LGALS3 using GCS-100 suppresses phosphorylation of ERK, the mechanism how LGALS3 supports MCL-1 in the AML cells may involve ERK. OCI-AML3 LGALS3 shRNA cells were more sensitive to ABT-737 compared to control cells. RPPA also revealed that LGALS3 was strongly correlated with phosphorylated PKC delta and suppression of LGALS3 by shRNA reduced PKC delta phosphorylation levels implicating LGALS3 as a positive regulator of the kinase. While PKC delta is viewed foremost as a stress kinase, RPPA analysis of phosphorylated (i.e. active) PKC delta in AML patients reveals that elevated levels of active kinase is a prognostic factor associated with poor survival. The pro-survival role of PKC delta in AML may reflect its function as an AKT kinase or as a suppressor of ROS. RPPA also identified a novel positive association of LGALS3 with CD74. OCI-AML3 cells with LGALS3 shRNA did not exhibit altered CD74 expression suggesting that CD74 is upstream of the galectin or possibly that an as yet unidentified protein serves as a common regulator of both LGALS3 and CD74. Stromal cell derived CD74 has been implicated as important in the AML microenvironment as a regulator of IL-8 production (Abdul-Aziz et al Cancer Research 2016) but the role for CD74 in AML blast cells is not clear. CD74 modulates CD44 function so the protein could participate in signaling cascades involving the receptor. Pearson correlation with the other proteins analyzed identified 23 proteins including CD44 and LGALS3 that were positively correlated with CD74 and 8 proteins that were negatively associated with CD74. Though LGALS3 was not positively correlated with CD44, the galectin could potentially modulate CD44 function by influencing its surface expression as LGALS3 is involved in trafficking CD44 to the cell surface. The protein associations of LGALS3 and CD74 were used to assemble protein clusters that were then used for analysis of patient survival. LGALS3 and CD74 were collectively analyzed as part of a group of T-cell regulating proteins analyzed on this array. Within that group 4 different expression patterns (clusters) were defined by hierarchical clustering with LGALS3 being active in 2 of them. The protein cluster identifying active LGALS3 was prognostic for shorter remission duration. AML patients who exhibited both LGALS3 and CD74 active clusters exhibited very poor survival characteristics. For AML patients with both LGALS3 and CD74 active protein cluster, median overall survival was only 24.3 weeks, median remission duration was 17.8 weeks, and no patient survived beyond one year. These data suggest that LGALS3 supports AML cell survival via diverse pathways including positive regulation of MCL-1 and PKC delta and support of a CD74 protein network that identifies a new high risk AML population. It is our hope that strategies targeting LGALS3 and CD74 can be developed to help this new at risk population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal